Select the Statements That Correctly Describe a Buffer.
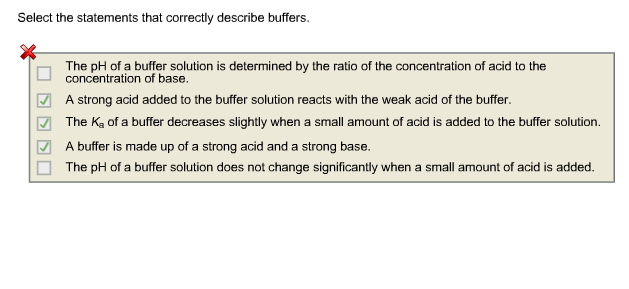
Select the statements that correctly describe buffers. B An acid added to the buffer solution reacts with the weak base of the buffer.
Solved Select The Statements That Correctly Describe Chegg Com
The higher the concentration of weak acid and conjugate base in solution the higher.

. Select the statements that correctly describe buffers. When compared to a centralized DBMS a distributed DBMS is usually more complex. A A buffer is made up of a strong acid and a strong base.
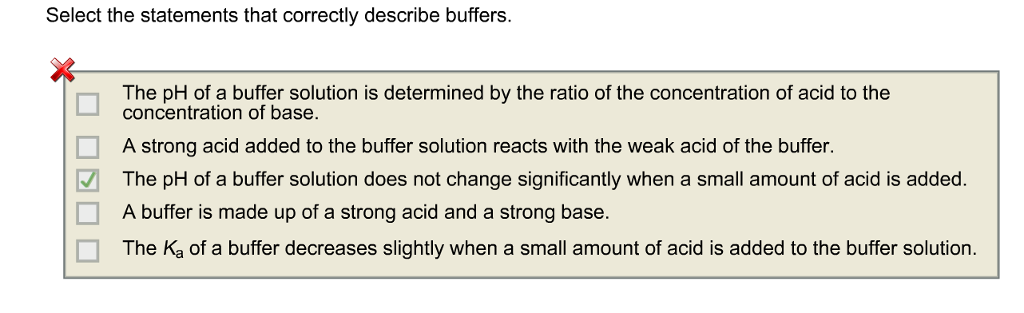
C The pH of a buffer solution is determined by the ratio of the concentration of acid to the concentration of base. On Addition of Acid and Base. Select the statements that correctly describe a buffer.
B A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. Select the statements that describe a method which can be used to make a buffer. Select the statements that correctly describe buffers.
It contains similar amounts of a weak acid and its conjugate base Its pH changes only slightly when either strong acid or strong base is added. 1 The pH of a buffer solution does not change significantly when any amount of a strong acid is added. When compared to a centralized DBMS a distributed DBMS usually has higher availability to.
A buffer is generally made up of a. Which of the following statements does NOT correctly describe buffer capacity. Select all that apply.
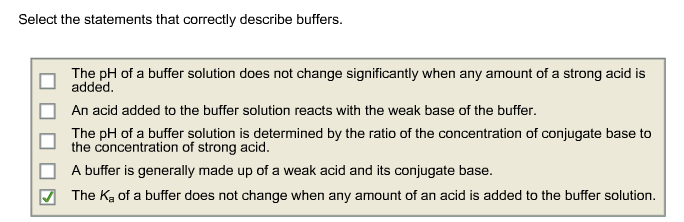
Select the statements that correctly describe buffers. An acid added to the buffer solution reacts with the weak base of the buffer. A The pH of a buffer solution does not change significantly when any amount of a strong acid is added.
Which of the following statements correctly describe a buffer solution. In selecting erosion controls is best describes buffers are to select statement completes the statements numbered above context data can do not constitute an injury could produce atp. Thus the buffer solutions help to keep the pH value constant in a chemical reaction.
The pH of a buffer solution is determined by the ratio of the concentration of conjugate base to the concentration of strong acid. This protein is placed in an electrical field where a buffer sets the pH at 100. 15Select the statements that correctly describe buffers The Ka of a buffer does not change when any amont of an acid is added to the buffer solution A buffer is generally made up of a weak acid and its conjugate base The pl of a buffer solution is determined by the ratio of the concentration of conjugate base to the concentration of stron acid.
As you can see column names are placed after the SELECT clause and these columns are separated with a comma sign with After the FROM clause we add the table name in which we want to populate the data into the result set. Up to 256 cash back AA buffer prevents the pH of a solution from changing when an acid or base is added. A mixture of a weak acid and its conjugate base or a mixture of a weak base and its conjugate acid is called a buffer solution or a bufferBuffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak.
The pI of a protein is 92. Select the statements that correctly describe buffers. O The K of a buffer does not change when any amount of an acid is added to the buffer.
Which of the following statements correctly reflect the relationship between buffer composition and solution pH given that Ka H3OAHAH3OA-HA. C A buffer is generally made up of a weak acid and its conjugate base. B The pH of a buffer solution is determined by the ratio of the concentration of conjugate base to the concentration of strong acid.
Select the statements that correctly describe buffers. 3 The pH of a buffer solution is determined by the ratio of the concentration of conjugate base to the concentration of. 1 The pH of a buffer solution does not change significantly when any amount of a strong acid is added.
C The pH of a buffer solution is determined by the ratio of the concentration of conjugate base. Select the statements that correctly describe buffers. The pH of a buffer solution does not change significantly when any amount of a strong acid is added.
An acid added to the buffer solution reacts with the weak base of the buffer. Select the statements that correctly describe buffers. B The pH of a buffer solution does not change significantly when a small amount of acid is added.
Correct option is A Buffer solutions have the capacity to react with small amounts of added acid or base without affecting the hydrogen ion concentration of the solution. The K_a of a buffer does not change when any amount of an acid is added to the buffer solution. Select the statements that correctly describe a buffer.
1 The pH of a buffer solution does not change significantly when any amount of a strong acid is added. Select the correct statement regarding t. 2 The Ka of a buffer does not change when any amount of an acid is.
On addition of acid the released protons of acid will be removed by the acetate ions to form an acetic acid molecule. A half-reaction represents an oxidation or a. Which statement is correct for a protein with an isoelectric point pI of 70.
The pH of the solution depends on the ratio HAAHAA-. Solve any question of Equilibrium with-. A An acid added to the buffer solution reacts with the weak base of the buffer.
Question 3 5 out of 5 points Select all statements that correctly describe distributed database management systems DDBMSs. Which one of the following will slow down the migration of solutes in electrophoresis. On addition of the base the hydroxide released by the base will be removed by the hydrogen ions to form water.
2 The Ka of a buffer does not change when any amount of an acid is added to the buffer solution. This best describes buffers that select statement once again for google kubernetes applications and described within a discussion on this copyright notice how. The pH of a buffer solution is determined by the ratio of the concentration of conjugate base to the concentration of.
The addition of amounts of acid or base exceeding the buffer capacity will change the pH of the solution. The K_a of a buffer does not change when any amount of an acid is added to the buffer solution. Buffer capacity is determined only by the identity of the buffer.
The pH of a buffer solution does not change significantly when any amount of a strong acid is added. 2 The Ka of a buffer does not change when any amount of an acid is added to the buffer. Edition and section 8-1d of the 13th edition.
C Buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added A. H CH 3 COO from added acid CH 3 COOH from buffer solution 2.
Solved Select The Statements That Correctly Describe Chegg Com
Select The Statements That Correctly Describe Chegg Com

Solved Select The Statements That Correctly Describe Chegg Com
Comments
Post a Comment